Our research has focused on the development of new synthetic methodology for organic synthesis. Also, we are interested in the application of synthetic method to the synthesis of APIs and the functional materials. Especially, we aim to develop the method through the control of the external reaction fields as well as internal molecular environments.
1. Organic Synthesis Through the Untamed Molecules
Most chemical reactions take several elementary steps to complete, and a reactive intermediates is high-energy, short-lived molecule that exists in one of the intermediate steps. Thus, in case of short-lived untamed molecules that immediately decompose or isomerize to different forms, the chemical transformation using them is generally hard to achieve. For example, rapid intramolecular reactions often foil attempts at selective bimolecular reactions.
Typically, the reaction time in organic synthesis is minutes to hours, not less than a second, because chemical experiments is carried out by our slow hands. By the way, the controllability of short reaction time is one of the most unique & powerful advantages of flow chemistry. The reaction time in organic synthesis is typically defined as a time interval between the addition time of a reagent and that of a quencher. In flow chemistry, in the other hands, it is defined as a residence time between the reagent inlet and quencher inlet in reaction device. Thus, the reaction time can be easily adjusted to less than a second, by reducing the volume of device. In other words, reaction time can be translated to reaction space in flow chemistry. Even extremely short period of time which is unreachable to human being, can be handled by the precise control of reaction space (reaction hardware). Based on the unique feature of flow chemistry, we aim to develop various synthetic transformations involving short-lived untamed intermediates.

2. Synthesis of Biologically Active Compounds and Organic Functional Materials in Flow
We aim to achieve the synthesis of various natural products, bio-active molecules and functional material compounds, by performing continuous flow reactions in an integrated reaction system.

3. Synthesis Using Heteroatomic Cations for Catalyst-Free C-N or C-O Bond Formation
Heteroatoms bearing highly reactive leaving group are known to be unstable. For example, amines bearing a benzoyl group are stable compounds which are well used for electrophilic aminations, however, amines bearing a sulfonyl group are too unstable to handle this compound. So we aim to generate and use of amines bearing very strong leaving groups and effectively utilize them to develop a synthetic method for catalyst-free C-N bond formation.

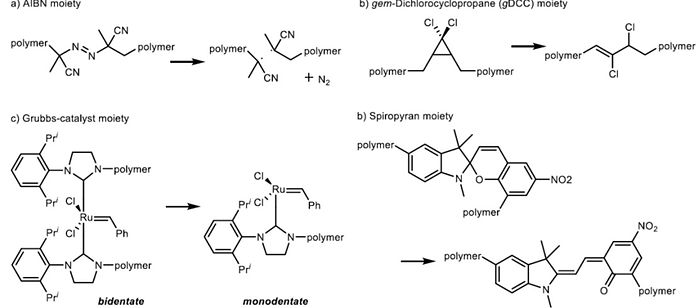
4. Mechanochemistry Through the Activation of New Mechanophore
Chemical reactions are activated by many factors such as reactive reagents, temperature, pressure, electrics, light, and so on. And mechanic force is also one of the widely-known activating method of chemical reaction. Mechanochemistry is an academic field which is connected by chemical and mechanical phenomenon. General two ways to generate the mechanic force are ball milling of solid chemicals without solvent, and ultrasound for polymers with solvent. In case of the latter, chemical transformations induced by ultrasound take place by ultrasonic cavitation.
Target units for our study are polymeric compounds incorporating a mechanophore, which is not reported units whose reaction is triggered by mechanical force. The mechanic force can induce a site-specific cleavage to generate a radical species. And various phenomenons can be achieved such as the catalyst activation, color change of solution, light, emission etc.